 |

|
>>>> Vitro
Retinal Products
– Micromed
|
|
|
|
 |
PDMS
- Silicone oil in a sterile
CLASSIFICATION
The medical device: PDMS silicone
oil for ocular implant is a Class
IIb medical devices under rule 8 of
Annex IX of Directive 93/42 EEC. Duration
Long term Surgically invasive device.
D Allocation of use
The PDMS and 'an oily liquid with
high viscosity' intended to replace
the vitreous humor in the speeches
of vitrectomy with long residence
time in the eye typically longer than
30 days.
Product
Overview
The PDMS and 'consists of Polidimetilsiloxano
fluid.
Formula: [-Si (CH3) 2 -O]
n ]
Refractive index: 1.405 @
24 º C
Density ': 0.965 g / ml @
24 º C
Viscosity ': 1000/2000/5000
centistokes @ 25 º C
And it 'available in sterile vials
7,10,15 and 20 cc.
|
|
|
|
|
|
Perfluorocarbon
liquid – sterile (Liquid Perfluoro Carbon
)
CLASSIFICATION : The medical
device: ( Liquid perfluorocarbon liquid perfluoro
Carbide Octodecafluorodecahydronaphtalene 98%
belongs to the class IIa medical device according
to Rule 7 of Annex IX of Directive 93/42 EEC.
Duration Transient. Surgically invasive device.
)
Intended Use : The perfluorocarbon
liquid Liquid Perfluoro Carbide and 'a high-density
liquid' intended to wash the vitreous chamber
during ocular surgery with maximum dwell time
in the eye of an hour.
Product Overview : The perfluorocarbon
liquid and 'constituted by Octadecafluorodecahydronaphthalene
(cis + trans) to 98%. Anhydrous 100% fluorinated
and hydrophobicImpurities' present are completely
fluorinated and are open fluorinated chains
of the same type.
Formula: C10F18
CAS No.: 306-94-5
EEC No.2061924
Refractive index: 1.314 @ 24
º C
Viscosity ': 2.7 centistokes
@ 25 º C
Relative molecular mass Mr:
462.08
Bleistein Registry Number BRN:
2067113
Boiling Point: 141-143 º
C
Density ': 1.941 g / ml @ 24
º C
|
|
|
|
MicroC3F8
- Octafluoropropane in sterile disposable pack
CLASSIFICATION : The
medical device: MicroC3F8 (Hexafluoroethane
99.95%, belongs to the class IIb medical devices
under rule 8 of Annex IX of Directive 93/42
EEC: Prolonged duration. Surgically invasive
device.)
Intended
Use The MicroC3F8and 'a gas with high molecular
weight is intended to replace the vitreous
humor in the interventions of vitrectomy,
with average residence time in the eye no
more than 28 days.
Product
Overview: The MicroC3F8 and 'consists
of C3F8 to 99.95% mixed with dry nitrogen
to 99.9%.
Name: octafluoropropane
Formula: [C3F8]
Molecular weight: 188
Melting point: -183 °
C
Boiling point: -36.7 º
C
Density 'on gas: 6.5
Density 'on liquid: 1.4
Vapour pressure at 20 º C:
7.7 bar
Solubility 'in water: not
available
Gas Appearance: colorless
Odor: ethereal,
CAS No. 00076-19-7,
EEC No. 200-941-9
Description: The MicroGas
are distributed non-sterile in aluminum cans
with spout and security seal, pure or mixed
with dry nitrogen. The product is well packaged:
1 sealed envelope containing the canister
and a 0.22µ Millipore filter (Millex
GV) with adapter for connection to the canister.
The exterior of the bag is sterilized by ethylene
oxide, 1 sterile syringe (CE marked), 1 two-way
cock sterile (CE marking), August 1 27G and
30G.
|
|
|
|
MicroSF6
- Sulphur hexafluoride in a sterile disposable
Pack
CLASSIFICATION : The medical device
(MicroSF6 Sulfur hexafluoride 99.9%, belongs
to the class IIb medical devices under rule
8 of Annex IX of Directive 93/42 EEC: Prolonged
duration. Surgically invasive device. )
Intended Use : The MicroSF6 and 'a gas with
high molecular weight is intended to replace
the vitreous humor in the interventions of vitrectomy,
with average residence time in the eye no longer
than 7 days.
Product Overview : The MicroSF6
and 'consists of SF6 99.9% mixed with dry nitrogen
to 99.9%.
Name: Sulphur hexafluoride
Formula: [SF6]
Molecular weight: 146
Melting point: -50.8 º
C
Boiling point: -64 º C
Density 'on gas: 5
Density 'on liquid 1.4
Vapour pressure at 20 ° C: 21
bar
Solubility 'in water: 41 mg
/ l Gas
Appearance: colorless
Odour: none,
CAS No. 02551-62-
EEC No. 219-854-2 |
|
|
|
Silibend
- Silicone rubber bands and implants - Sterile
disposable
Classification : The medical
device (SiliBend lens implants made of silicone
rubber scleral surgery for a Class IIb medical
devices under rule 8 of Annex IX of Directive
93/42 EEC. Long-term durability. Surgically
invasive device )
Intended Use : The SiliBend
are strips of silicone rubber of various shapes
used to bind the eyeball to the level of the
sclera in order to limit the retina where
it was detached.
Composition : 84% dimethylpolysiloxane
compound translucent with inert fillers 16%,
0.0124% isopropyl carbonate, carbonate perossitertiobutile
0006. |
|
| Product
Range |
| Code
|
N
° Cat |
Labtician
|
Target
|
Storz |
Measure
|
Track
Width |
Section
|
| BPP1
(**) |
MMD-701
|
S2969
|
S5-1001
|
E
5381 600 |
2x0,
8 mm |
- |
 |
| BP1
(**) |
MMD-703
|
S2987 |
S5-2001
|
E
5381 700 |
2.5
x0, 7 mm |
- |
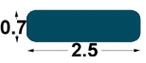 |
| BM1
|
MMD-705
|
S2970 |
S5-1011
|
- |
3.5
x0, 9 mm |
- |
 |
| BL1
|
MMD-707
|
S2971
|
S5-1021
|
E
5381 801 |
4.2
x1, 1 mm |
- |
 |
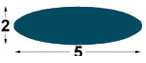
| IMP1
|
MMD-717
|
- |
- |
- |
5x2
mm |
- |
 |
| IMPP1 |
MMD-722
|
- |
- |
|
4x1,
5 mm |
- |
 |
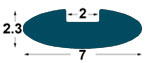
| IM1 |
MMD-709
|
S3014
|
S5-2311
|
|
7x2,
3 mm |
2
mm
(*) BPP1-BP1
|
 |
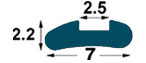
| IMA1
|
MMD-719
|
- |
|
|
7
mm-R1 3 |
2.5
mm
(*) BPP1-BP1
|
 |
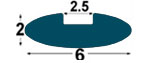
| IMM1 |
MMD-720
|
- |
|
|
6x2
mm |
2.5
mm
(*) BPP1-BP1
|
 |
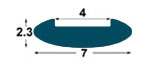
| IMSL
|
MMD-721
|
- |
|
|
7x2,
3 mm |
4
mm
(*) BPP1-BP1-BM1-BL1
|
 |
|
|
| For
Further Details Visit : http://www.micromed.it |
|
|
|
|

